Petroleum is created over millions of years from the decomposition of marine. As these organisms perish, they descend to the ocean’s depths. The sea floor mixes with sediments and is buried. Over time, they are subjected to heat and pressure, transforming into kerogen and, eventually, hydrocarbons in a process that includes sedimentation, burial, diagenesis, catagenesis, and metagenesis. The resulting oil migrates through the Earth’s crust until it’s trapped by rock formations, creating oil reservoirs.
In refining, crude oil is first separated in a distillation column, heating the oil to different temperatures to boil off various hydrocarbon chains at different points. Lighter components like propane and butane are collected at the top, while heavier ones settle lower. Some heavy fractions undergo conversion processes, such as cracking, to break down large molecules, making them more useful. Impurities are removed, and finally, the products are blended to meet quality standards for various uses like gasoline or diesel. This refining process converts raw crude into various essential products tailored to serve specific purposes in industry and everyday life.
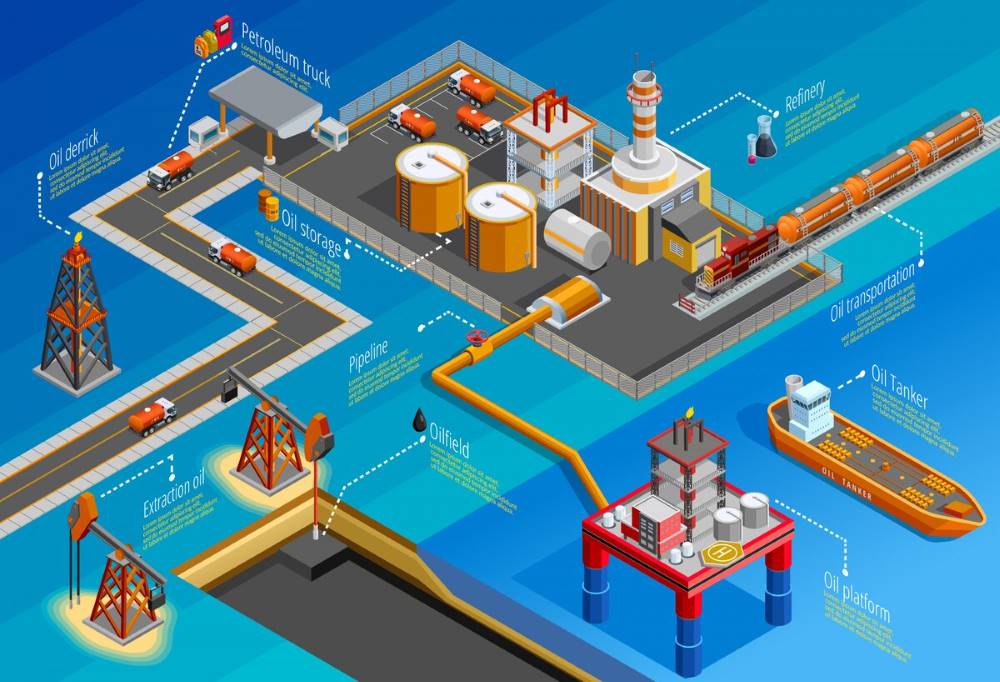
What is petroleum?
Crude oil, referred to as petroleum, is a naturally derived, combustible liquid composed of an intricate assortment of hydrocarbons with varying molecular masses and additional organic elements. Organic compounds. This substance is located within geological strata below the surface of the Earth. Petroleum and crude oil are extracted through oil drilling. Petroleum formation occurs over millions of years derived from the fossilized remains of deceased flora and fauna, primarily plankton, that settled in large quantities in the sea beds. Under layers of sediment and rock, this organic matter undergoes thermal breakdown processes due to high temperatures and pressure, transforming it into petroleum.
Petroleum is a non-renewable resource and a primary energy source globally. It is a crucial raw material for many chemical products, including pharmaceuticals, solvents, fertilizers, pesticides, and plastics. The versatility of petroleum comes from its ability to be refined into different products and its high energy density, which makes it a powerful fuel source. Refining petroleum separates it into components used for various applications, from transportation fuels like gasoline and diesel to heating oil and feedstocks for the petrochemical industry. Its importance to modern society is unparalleled, making it a cornerstone of the global economy.
Formation of Petroleum
Petroleum formation is a geological process that spans millions of years, transforming organic material into the oil reserves we exploit today. It begins with accumulating dead microscopic organisms, primarily algae and zooplankton, in marine environments. As these organisms settle on the ocean floor, they mix with sediment, forming a sludge. Over time, additional sediment layers bury this organic-rich material, isolating it from oxygen and subjecting it to increasing pressure and heat.

This environment initiates a series of chemical reactions, slowly converting the biomass into kerogen, a waxy substance in various oil shales worldwide. With further burial, the temperature rise pushes the kerogen through a process known as catagenesis, breaking it down into smaller, liquid hydrocarbons, crude oil and gaseous hydrocarbons, and natural gas.
Petroleum Refining Process
Petroleum refining is a multi-step industrial process that converts crude oil into useful products.
Crude oil, a multifaceted blend of hydrocarbons and other chemicals, is first transported to refineries. Here, it undergoes a primary separation process in a distillation column, where it is heated. As the temperature rises, different hydrocarbon compounds boil off. They are recovered at various points along the column, separating the crude into fractions such as naphtha, kerosene, diesel, and residues based on boiling points.

Subsequent processing tailors these fractions to meet market demands or to further refine them. This involves conversion techniques like catalytic cracking, where heavy molecules are broken into lighter ones; alkylation, which combines small molecules into larger, more complex ones; and reforming, which rearranges molecular structures to improve fuel quality.
The treatment phase removes impurities, such as sulfur, using methods like hydrodesulfurization. The refined products are then subject to blending, creating the exact types of fuels needed for various applications—gasoline with specific octane levels, jet fuel, or diesel. This intricate series of processes ensures that crude oil is transformed into high-quality, market-ready fuels and materials.
Pros and Cons of Petroleum Refining and Formation Process
Pros:
- Economic Growth: Petroleum is a cornerstone of the global economy, underpinning industries and providing employment.
- Energy Density: It has a big energy density, delivering much energy per volume.
- Versatility: Refined petroleum products support a variety of essential uses, from fuels to manufacturing chemicals.
- Infrastructure: Existing infrastructure is well-developed, facilitating efficient distribution and consumption.
Cons:
- Environmental Impact: Extraction and use of petroleum contribute significantly to pollution and are major sources of greenhouse gases leading to climate change.
- Resource Depletion: Petroleum is a non-renewable resource, with reserves finite and declining as consumption continues.
- Economic Volatility: Oil prices are volatile, impacting global economies.
- Oil Spills: Accidental spills during extraction and transportation can have devastating ecological consequences.
- Dependency: Overreliance on petroleum can stifle the development of renewable energy sources and leave economies vulnerable to supply disruptions.
Petroleum Refining Process Flow Chart
A petroleum refining process flow chart typically outlines the journey of crude oil from its raw state to the various end products. The chart starts with the Incoming Crude Oil, which is first stored in tanks before entering the refining process.

The initial stage is Distillation, where the crude oil is heated in a distillation tower. As the temperature rises, different hydrocarbons vaporize at different temperatures. They are collected in trays at various heights in the column, resulting in products like light gases, naphtha, kerosene, diesel, and heavy residue.
From here, the flow chart branches out:
- Light gases go to Gas Recovery for further processing into propane and butane.
- Light distillates such as naphtha move to Catalytic Reforming to enhance octane numbers or to Petrochemical Synthesis as feedstock.
- Middle distillates like kerosene and diesel are polished in Hydrotreating Units to remove sulfur.
- Heavy residue undergoes Conversion Processes like cracking to break down into lighter, more valuable products.
Each stream may go through Additional Treatment to remove impurities or improve quality. The final step is Blending, where refined products are combined in precise proportions to achieve specific standards before being sent out as Finished Products like gasoline, jet fuel, and diesel.
Products produced in Petroleum Refining Process
- Gasoline: Used as fuel in vehicles, it’s the most well-known product of crude oil refining.
- Diesel: Another transportation fuel, it’s used in engines that require more torque and are often found in trucks and buses.
- Jet Fuel: Specialized fuel for aircraft.
- Fuel Oils: Used for heating and powering ships.
- Lubricants: Reduce friction in engines and machinery.
- Asphalt: Used in road construction.
- Petrochemical Feedstocks: Basis for plastics, synthetic rubber, solvents, and additional chemicals.
- Liquefied Petroleum Gas (LPG): Propane and butane are utilized as an energy source in heating devices, culinary tools, and modes of transportation.
- Kerosene: Used as fuel in lamps and heaters.
Each product has specific quality standards and is tailored for its particular market, from consumer-level use in vehicles and homes to industrial applications in manufacturing and transportation.